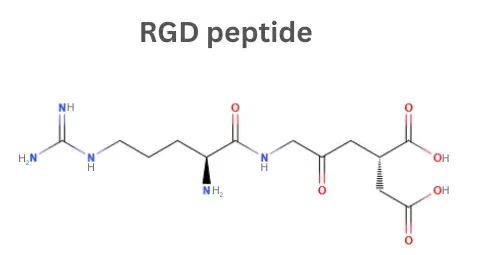
RGD Peptides: Integrin Binding Motifs for Cell Adhesion
The RGD peptide, also known as arginine–glycine–aspartic acid tripeptide or arginylglycylaspartic acid, is one of the most widely studied cell-adhesive sequences. Naturally present in many extracellular matrix and serum proteins, the RGD motif binds to integrins, transmembrane receptors expressed on many cell types and often overexpressed on cancer cells. Consequently, RGD-containing peptides are widely used to functionalise biomaterials and to improve the targeting of drug delivery and imaging agents, enabling controlled cell adhesion and more specific cellular interactions. Their reproducibility, modularity, and compatibility with diverse substrates make them valuable tools in tissue engineering, regenerative medicine, cellular agriculture, and cancer research.
To support research in these areas, we offer a collection of RGD peptides or can custom-synthesise them to your specifications. We can also quantify peptide concentrations in biomaterials using our ISO 17025:2017 amino acid analysis service. Contact us to find out more.
RGD Peptides: Key Integrin-Binding Ligands
What are integrins?
Integrins are proteins on the cell surface that help cells attach to their surroundings and communicate with them. By linking the extracellular matrix (ECM) to the cell’s internal structure, integrins allow cells to sense their environment and respond through movement, growth, or survival. They play a key role in processes like wound healing, immune responses, and development.
Discovery of the RGD motif
In 1984, Pierschbacher and Ruoslahti demonstrated that short synthetic peptides containing the RGD sequence were sufficient to inhibit cell attachment to fibronectin, a major ECM protein. Their work established RGD as the minimal recognition motif required for integrin-mediated cell adhesion. Subsequent studies identified RGD sites in additional ECM proteins, including vitronectin and fibrinogen, highlighting the broad significance of this tripeptide in mediating cell–matrix interactions.
Applications of RGD Peptides
Biomaterial and Scaffold Functionalisation
RGD peptides are widely used to functionalise biomaterials and scaffolds, addressing the lack of natural cell-adhesive signals in many synthetic polymers. By introducing integrin-binding motifs, RGD conjugation promotes cell adhesion, enabling better interaction between cells and the material.
Common substrates include PEG, alginate (a polysaccharide derived from brown algae), silk fibroin, gelatin methacrylate (GelMA), PLGA (poly(lactic-co-glycolic) acid) and synthetic polyesters like PLA, and PCL, where covalent attachment ensures stable presentation of RGD without affecting the material’s structure or strength.
As a result, RGD-functionalised scaffolds provide reliable platforms for:
- Creating organised tissue-like constructs, including bone, heart, blood vessel tissues, and cornea. These scaffolds guide cells to grow and arrange in the desired structure, supporting the formation of functional tissue.
- Acting as temporary bioactive matrices that speed up healing by encouraging skin and connective tissue cells to migrate, stick, and grow.
- 3D cell culture systems and bioinks, enabling precise spatial organisation of cells in hydrogels or printed constructs.
Similarly, in emerging fields such as cellular agriculture, cultivated meat scaffolds like RGD-alginate hydrogels have been shown to facilitate the adhesion of C2C12 myoblasts to the biomaterial. Therefore, by decorating edible or biodegradable scaffolds with RGD motifs, muscle progenitor cells can better attach, spread, and differentiate within 3D structures, helping to build organised tissues that more closely resemble conventional meat in texture and structure.
Precision Therapeutics and Diagnostics
RGD peptides also play a key role in precision therapeutics and diagnostics. They are commonly conjugated to nanoparticles, liposomes for targeted drug delivery, preferentially accumulating in integrin-rich tissues such as tumours or inflamed blood vessels.
When conjugated to fluorescent dyes, radiotracers, or MRI contrast agents, RGD peptides enable non-invasive visualisation of integrin-expressing tissues, improving tumour detection, angiogenesis monitoring, and treatment response assessment. Cancers including glioblastoma, melanoma, and breast tumours frequently overexpress integrins such as αvβ3 and αvβ5, making them ideal targets for these RGD-based peptides.
Advantages of RGD peptides
Compared to full-length extracellular matrix (ECM) proteins, which are structurally complex and costly, or small-molecule ligands, which often lack physiological relevance, RGD provides specific integrin-binding cues that regulate cell adhesion, spreading, migration, and survival.
Other key advantages of RGD peptides include:
- Reduced Immunogenicity: Compared with animal-derived ECM proteins, synthetic RGD peptides are chemically defined, consistent across batches, and carry a lower risk of immune reactions or contamination.
- Thermal Stability: RGD peptides generally retain bioactivity after common sterilisation methods, such as autoclaving, whereas full-length proteins can denature, losing functionality.
- Precision Control Over Ligand Presentation: Peptide density, spatial orientation, and multivalency can be finely tuned, allowing researchers to regulate integrin clustering and downstream cellular signalling in a predictable manner.
Choosing the Right RGD Peptide Variant
Practical Limitations: Stability and Off-Target Considerations
Selecting the appropriate RGD peptide variant depends on the required stability, integrin specificity, and mode of presentation in the target application. While RGD peptides are highly versatile, differences in structure and sequence strongly influence their biological performance in vitro and in vivo.
Linear RGD peptides are susceptible to proteolytic degradation, which can limit their functional lifetime in biological environments. Structural modifications such as peptide cyclisation or incorporation of D-amino acids significantly improve protease resistance and extend bioactivity. In addition, because the RGD motif is recognised by multiple integrins (including αvβ3, αvβ5, and α5β1) non-specific or off-target interactions may occur. These effects can be mitigated through careful design of peptide sequences.
Linear and Cyclic RGD Peptides Variants
Beyond the minimal RGD tripeptide (for example RGDS, the standard positive control for cell adhesion assays), a range of extended and modified RGD sequences have been developed to enhance affinity, selectivity, and functional stability. Synthetic RGD peptides typically act as high-affinity integrin ligands with nanomolar IC₅₀ values, and further optimisation can be achieved through cyclisation, the addition of flanking residues, or multivalent presentation.
- Linear peptides such as GRGDSP and GRGDNP incorporate flanking amino acids that improve water solubility and strengthen integrin binding compared with minimal RGD sequences. These peptides are widely tethered to hydrogels, scaffolds, and biomaterial surfaces to promote controlled cell adhesion and spreading in tissue engineering and in vitro culture systems.
- Cyclic RGD peptides, including c(RGDfK), c(RGDfC), and cilengitide ((c[RGDf(NMe)V]), EMD 121974) generally outperform linear variants by constraining the RGD motif into a rigid β-turn conformation that closely matches the integrin binding pocket. Incorporation of D-phenylalanine increases protease resistance, while cyclisation enhances binding affinity by up to 100–1000-fold. c(RGDfK) is commonly used for nanoparticle conjugation via its lysine side chain, whereas c(RGDfC) enables thiol-based chemistries for imaging and surface modification. Cilengitide has higher affinity for the αvβ3 integrin compared with linear analogues such as GRGDS, making it a benchmark compound for integrin-targeted therapeutic development.
- For applications requiring enhanced tissue penetration, the cell-penetrating peptide iRGD (CRGDKGPDC) provides an additional level of targeting. This tumour-homing cyclic peptide initially binds integrins on tumour vasculature through its RGD motif. Following proteolytic cleavage, a cryptic CendR sequence is exposed, enabling binding to neuropilin-1 and triggering deep tissue penetration, thereby improving drug delivery into solid tumours.
The following table summarises common RGD peptides, their type (linear or cyclic), the integrins they primarily bind to, and their typical applications in research and biotechnology.
| RGD Peptide | Type | Primary Integrin Targets | Typical Applications |
| RGD | Linear | α5β1, αvβ3, αvβ5 | Basic adhesion studies |
| RGDS | Linear | α5β1, αvβ3, αvβ5 | Adhesion assays |
| RGDT | Linear | α5β1, αvβ3 | Experimental adhesion studies |
| RGDV | Linear | αvβ3, αvβ5 | Cell adhesion, integrin studies |
| RGDFV | Linear | αvβ3, αvβ5 | Biomaterial coating, cell adhesion |
| RGDR | Linear | α5β1, αvβ3 | Cell adhesion |
| RGDGR | Linear | α5β1, αvβ3 | In vitro adhesion studies |
| RGDFK | Linear | αvβ3, αvβ5 | Targeting, conjugation |
| RGDFK | Cyclic | αvβ3, αvβ5 | Targeting, conjugation |
| GRGDS | Linear | α5β1, αvβ3, αvβ5 | Cell adhesion, hydrogel functionalization |
| GRGDSP | Linear | α5β1, αvβ3, αvβ5 | Tissue engineering scaffolds |
| GRGDNP | Linear | α5β1, αvβ3 | In vitro culture surfaces |
| GRGDY | Linear | α5β1, αvβ3, αvβ5 | Cell adhesion assays |
| YRGD | Linear | α5β1, αvβ3, αvβ5 | Functionalized biomaterials |
| GRGDSY | Linear | α5β1, αvβ3, αvβ5 | Cell adhesion, in vitro culture |
| KYGRGDS | Linear | α5β1, αvβ3, αvβ5 | Biomaterial functionalization |
| GGGGRGDSP | Linear | α5β1, αvβ3, αvβ5 | Hydrogel surfaces, biomaterials |
| c(RGDfC) | Cyclic | αvβ3, αvβ5 | Surface modification, imaging |
| c(RGDyK) | Cyclic | αvβ3, αvβ5 | Imaging, nanoparticle targeting |
| cRGD | Cyclic | αvβ3, αvβ5 | General integrin-targeting applications |
| RGD4C | Cyclic | αvβ3, αvβ5 | Phage display, targeting studies |
| CCRGDKGPDC | Cyclic | αvβ3, αvβ5 (initial), neuropilin-1 (after cleavage) | Drug delivery, tumour targeting |
Alternative Cell Adhesion Peptides and Complementary Motifs
While RGD peptides promote general cell adhesion via integrins, other peptide motifs can enhance scaffold performance by targeting specific integrins or cellular pathways. In particular,
- PHSRN (fibronectin-derived) synergises with RGD to increase stem cell adhesion and proliferation.
- GFOGER (triple helical collagen mimetic peptide) specifically binds α2β1 integrins, supporting mesenchymal stem cell (MSC) attachment.
- Laminin-derived sequences such as IKVAV and YIGSR promote cell adhesion, spreading, and differentiation in myogenic and fibroblast cells.
The addition of complementary motifs alongside RGD can further improve cell migration, tissue organisation, and functional tissue formation. Using multiple adhesion peptides also allows scaffold designs to achieve cell-type-specific adhesion and enhance tissue functionality beyond what RGD alone provides.
Why Analyse Biomaterials Containing RGD Peptides?
Formation of RGD-Modified Biomaterials
RGD peptides can be incorporated into biomaterials using physical or chemical strategies. Physical adsorption allows peptides to bind weakly to the surface of 2D films or the internal surfaces of 3D scaffolds. Alternatively, peptides can be chemically immobilised or grafted through covalent bonds to polymer functional groups, improving stability and controlled presentation.
These methods allow Arg-Gly-Asp motifs to be presented on flat coatings, within porous 3D structures, or as part of polymer backbones, enabling tailored cell-adhesive properties for specific biomaterial designs and applications.
Quantification and Validation of RGD Peptides Incorporation
Reliable characterisation of peptide incorporation is essential to ensure reproducible bioactivity. Because RGD is often immobilised at low densities within complex matrices, detection can be challenging. Amino acid analysis is one of the most robust methods for quantifying RGD content. By hydrolysing the biomaterial and measuring the molar abundance of arginine, glycine, and aspartic acid, researchers can confirm successful incorporation and determine ligand density.
Key advantages of amino acid analysis include:
- Compatibility with challenging or embedded peptide systems
- Ability to distinguish between covalently bound and non-specifically adsorbed peptides when combined with appropriate washing or control samples
- Rigorous assessment of conjugation efficiency
Complementary Analytical Approaches
Other techniques can support RGD quantification and visualisation:
- Fluorescent or dye-labelled peptides, which allow both quantification and imaging of immobilised peptides
- Radio-labelling for measuring peptide content and coupling efficiency
- Chromatography, spectroscopy, and ELISA for solution or surface peptide assessment
By combining amino acid analysis with these complementary techniques, researchers can ensure precise and reproducible incorporation of RGD peptides into biomaterials, supporting both quantitative and visual validation, including fluorescent labelling for specialised applications.
High Quality RGD Peptides for All your Research Needs
At AltaBioscience, we offer a large collection of RGD peptides or can synthesise them with custom modifications, including cyclic peptides, fluorescently labelled peptides, and PEG-conjugated peptides.
We also offer quantification of peptide incorporation into biomaterials using our ISO 17025:2017 accredited amino acid analysis service. Our team has long-standing expertise in analysing complex peptide–polymer conjugates, including low-concentration peptide systems and challenging materials such as RGD-alginate biomaterials that are prone to charring during hydrolysis.
For more information, contact us at info@altabioscience.com to discuss your project.
Summary
RGD peptides are short cell-adhesive sequences that mimic natural extracellular matrix signals. By bridging inert materials with ECM-like functionality, they enhance tissue engineering and regenerative medicine applications, including cultivated meat scaffolds. Their structural versatility (e.g., linear, cyclic, or multifunctional variants) allows fine-tuning of stability, binding affinity, and specificity.
In drug delivery and imaging, RGD motifs actively target integrin-overexpressing tissues such as tumours, enhancing nanoparticle or drug homing, cellular uptake, and precise visualisation.
Amino acid analysis of RGD peptides is commonly performed to verify composition, sequence integrity, and purity, ensuring consistent functional performance in both research and applied biotechnology.
Overall, RGD peptides combine biological functionality, material compatibility, and targeting capability, making them a powerful tool in both biomedical research and applied biotechnology.
References
Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3-9;309(5963):30-3. doi: 10.1038/309030a0. PMID: 6325925.
Alipour M, Baneshi M, Hosseinkhani S, Mahmoudi R, Jabari Arabzadeh A, Akrami M, Mehrzad J, Bardania H. Recent progress in biomedical applications of RGD-based ligand: From precise cancer theranostics to biomaterial engineering: A systematic review. J Biomed Mater Res A. 2020 Apr;108(4):839-850. doi: 10.1002/jbm.a.36862. Epub 2019 Dec 30. PMID: 31854488.
C. Bomkamp, S. C. Skaalure, G. F. Fernando, T. Ben-Arye, E. W. Swartz, E. A. Specht, Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2022, 9, 2102908. https://doi.org/10.1002/advs.202102908
Cossu J, Thoreau F, Boturyn D. Multimeric RGD-Based Strategies for Selective Drug Delivery to Tumor Tissues. Pharmaceutics. 2023 Feb 4;15(2):525. doi: 10.3390/pharmaceutics15020525. PMID: 36839846; PMCID: PMC9961187.
Kapp, T., Rechenmacher, F., Neubauer, S. et al. A Comprehensive Evaluation of the Activity and Selectivity Profile of Ligands for RGD-binding Integrins. Sci Rep 7, 39805 (2017). https://doi.org/10.1038/srep39805
Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem. 2010 Dec;10(10):753-68. doi: 10.2174/187152010794728639. PMID: 21269250; PMCID: PMC3267166.
Production and performance of biomaterials containing RGD peptides
Perlin, L., et al. Soft Matter, 2008, 4, 2331–2349. DOI: 10.1039/B801646A b801646a 2331..2349
Poreba, M. (2020), Protease-activated prodrugs: strategies, challenges, and future directions. FEBS J, 287: 1936-1969. https://doi.org/10.1111/febs.15227
Malcor, J.-D., & Mallein-Gerin, F. (2022). Biomaterial functionalization with triple-helical peptides for tissue engineering. Acta Biomaterialia, 148, 1–21. https://doi.org/10.1016/j.actbio.2022.06.003