Fmoc Amino Acids for SPPS
Fmoc solid phase peptide synthesis (SPPS) is the method of choice for peptide synthesis. Compared to the traditional Boc/Benzyl approach, Fmoc chemistry is a convenient and milder method for the manufacture of peptides. In addition, the availability of highly pure and inexpensive Fmoc amino acids bearing orthogonal side chain protecting groups, and the suitability of the technique for automation and parallel synthesis have contributed to its wide adoption by scientists in academia and industry alike. Today, the use of Fmoc chemistry enables the rapid and efficient synthesis of peptides, including those over 50 amino acid residues and complexity, making it a highly valuable tool for life science research.
Why is it necessary to use protected amino acids?
SPPS is a step-by-step process where a peptide is built one amino acid at a time. Indeed, amino acids consist of a central carbon atom bonded to an amino group (−NH2), a carboxyl group (−COOH), and a variable side chain (R group). This feature allows amino acids to link together by forming an amide bond between the amino group of the growing peptide anchored to an insoluble resin with the activated carboxylic acid moiety of the incoming amino acid. However, the reaction of the peptide on the resin with an unprotected amino acid usually results in unwanted side reactions and polymerisation. As a result, protection of the alpha-amino group is key to ensuring the amino acid does not react with itself or with intermediaries during the synthesis process.
Sat Sandhu, Head of Peptide Chemistry at AltaBioscience, explains: “Protection of the alpha-amino group is essential to ensure that the desired peptide bonds form selectively and to achieve high peptide yield and purity. Ideally, the protecting group should make the amino acids soluble in most organic solvents, be stable under coupling reaction conditions, be easily cleaved and minimise unwanted side reactions and epimerisation.”
In peptide synthesis, t-butyloxycarbonyl (Boc) or 9-fluorenylmethoxycarbonyl (Fmoc) are carbamate-protecting groups commonly used to protect the amine group of amino acids. Boc and Fmoc-protected amino acids, whether natural, unnatural or modified, are nowadays widely commercially available.
What are the advantages of using Fmoc amino acids?
The Fmoc protecting group was developed by Carpino and Han in 1970 and, since the 90s, has been commonly used in solid-phase peptide synthesis. While Boc SPPS can still be favoured for the synthesis of “difficult sequences” and peptides prone to aggregation, most peptides are usually synthesised using the Fmoc approach. Below are some benefits of Fmoc that explain its unabated use for the protection of amino acids and the synthesis of peptides:
- Milder peptide synthesis reaction conditions
One of the main factors that contributed to the popularity of Fmoc SPPS was the milder reaction conditions required for the cleavage of the peptide from the resin. In the Boc approach, researchers used liquid hydrogen fluoride (HF) and, as a result, required specialist equipment to circumvent its highly corrosive nature. On the contrary, Fmoc chemistry uses milder cleavage conditions with trifluoroacetic acid (TFA), which users usually prefer. Fmoc chemistry is also particularly advantageous and compatible with the synthesis of peptides with post-translational modifications, such as phosphorylated and glycosylated peptides. These were previously inaccessible under the harsher Boc reaction conditions.
- True side chain protecting group orthogonality
The removal of the alpha-amino protecting group is a crucial step in peptide synthesis. One of the main drawbacks of the traditional Boc approach was the partial loss of the side chain protein groups after repeated treatment of the growing peptide with TFA. Conversely, Fmoc has the advantage to be acid stable, but to be easily removed with a base. This feature allowed true orthogonality since the side chain protecting groups on the amino acids are acid-labile.
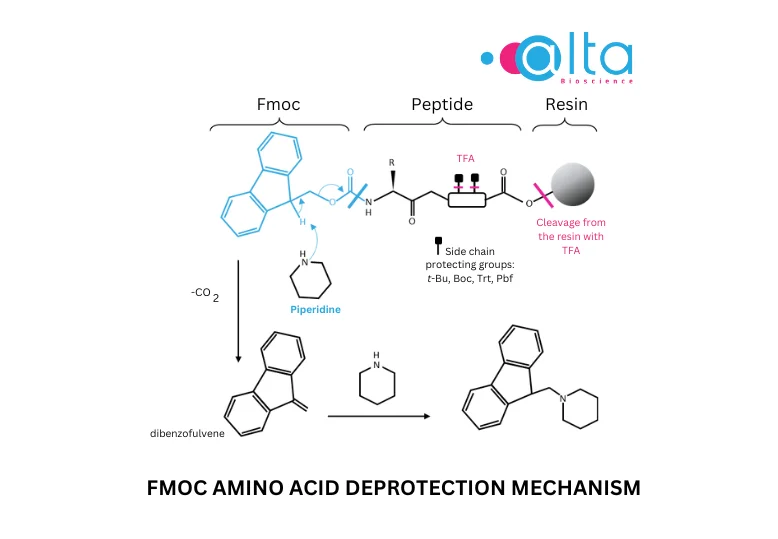
- Easy Fmoc deprotection
Fmoc deprotection involves the use of a weak amine base. The reaction tends to be slow with tertiary amines but more rapid with primary and secondary amines. Reactions in polar solvents also lead to faster reaction rates. Typical Fmoc deprotection conditions are 20% piperidine in DMF. Its mechanism can be described as follows: after deprotonation of the acidic proton by the base, a ß-elimination reaction occurs, producing carbon dioxide and the reactive dibenzofulvene by-product. The latter is scavenged by an excess of base to give a stable fulvene-piperidine adduct, which is removed during the washing steps.
- Easy reaction monitoring
The Fmoc group has the advantage of having strong UV absorbance. When performing reactions on solid support, researchers can exploit this useful feature to monitor the completion of coupling and deprotection reactions by photometry.
- Low cost of Fmoc building blocks
The development and large-scale synthesis of peptide therapeutics for a variety of indications has been a contributing factor in the manufacture of highly pure Fmoc amino acids. Due to the economies of scale in their production, Fmoc amino acids are now available at a low cost.
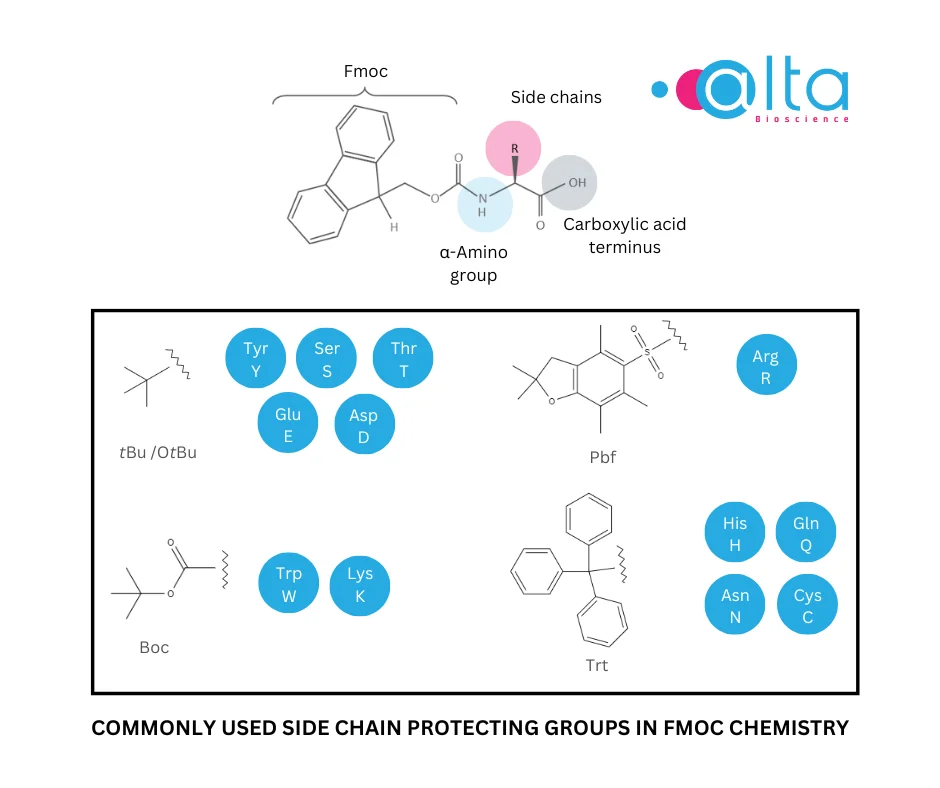
Which side chain protecting groups are compatible with Fmoc peptide chemistry?
Amino acids bear a variety of side chains with many reactive functionalities (e.g., amine, carboxylic acid, hydroxyl, thiol, imidazole, indole, guanidinium). The protection of these functional groups is key to ensuring high peptide yield and reducing the chance of unwanted side reactions. In Fmoc chemistry, side chain protecting groups must be:
- Orthogonal,
- Stable under the conditions used for Fmoc removal (typically mild bases like piperidine),
- Labile under the final deprotection conditions, usually involving strong acids like trifluoroacetic acid (TFA).
Below are commonly used side chain protecting groups in Fmoc chemistry:
Amino acids | Amino acid symbols | Side chain protecting group | Fmoc building blocks |
Arg | R | Pbf | |
Asn | N | Trt | |
Asp | D | OtBu | |
Cys | C | Trt | |
Gln | Q | Trt | |
Glu | E | OtBu | |
His | H | Trt | |
Lys | K | Boc | |
Ser | S | tBu | |
Trp | W | Boc | |
Thr | T | tBu | |
Tyr | Y | tBu |
High-quality Fmoc amino acids ready to ship
AltaBioscience supplies all 20 Fmoc protected amino acids globally, ensuring high-quality and reliable access to essential building blocks for SPPS worldwide. Our products undergo stringent quality control to ensure they meet and exceed industry standards. High-quality, pure Fmoc amino acids result in higher yields, easier peptide purification, a more consistent impurity profile in crude peptides, and smoother optimisation during scale-up. This reduces regulatory issues during GMP peptide manufacture, minimises synthesis failures, and simplifies troubleshooting, giving you confidence in the integrity of the amino acid building blocks.
Our ISO 9001-certified lab can also provide custom peptide synthesis services, ensuring your research needs are met with the highest quality standards.
References
Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4–27. doi: 10.1002/psc.2836
Li, W., O’Brien-Simpson, N.M., Hossain, M.A., & Wade, J.D. (2020). The 9-Fluorenylmethoxycarbonyl (Fmoc) Group in Chemical Peptide Synthesis – Its Past, Present, and Future. Australian Journal of Chemistry, 73, 271-276. doi:10.1071/ch19427