Cyclic peptides: Examples, Synthesis and Applications
Cyclic peptides are a unique class of organic compounds, characterised by a circular structure, which distinguishes them from their linear counterparts. Found naturally in many organisms, they have generated high levels of interest in the scientific community due to their remarkable chemical and biological properties. In this article, we provide an overview and look at the main strategies for their synthesis, and their applications. Additionally, we provide a glossary of key technical terms, highlighted in bold throughout the article.
What are cyclic peptides?
Cyclic peptides are a class of peptides with a circular (or macrocyclic) structure. They are widely found in nature, and have been isolated from microorganisms, plants and animals alike. They have a wide array of structures ranging from small cyclic dipeptides to large macrocycles bearing multiple loops. This is because their biosynthesis occurs via two main pathways – ribosomal and non-ribosomal.
What makes them particularly interesting and has spurred interest among scientists is their wide variety of biological functions, such as antibacterial, antifungal and anticancer activities. For instance, cyclosporine A, a cyclic polypeptide isolated from filamentous fungi, is an immunosuppressant currently used for solid organ transplantation. Nisin is another cyclic peptide example widely used as food preservative. It is a polycyclic antibacterial peptide produced by the bacterium Lactococcus lactis. These are just two well-known examples, but there are many others. To view more, we recommend visiting the following databases: CyclicPepedia knowledge base[1] and PepTherDia[2].
Cyclic peptide synthesis
In addition to being isolated in nature or expressed in the laboratory, they can also be synthesised through chemical methods. One of the main advantages of synthetic cyclopeptides is the possibility to incorporate modifications and peptide conjugation options. Furthermore, Fmoc-protected amino acids and other modified residues that can enable ring formation, are widely commercially available. This is advantageous as it allows for the synthesis of a broad range of macrocyclic structures with natural and non-natural linkages.
So, how are cyclic peptides made in the laboratory? From a retrosynthetic perspective, circular peptides can be synthesised following two main steps: first, the synthesis of the linear peptide chain followed by a ring closure step. The latter, known as macrocyclisation, is usually achieved using three main strategies:
- Head-to-tail (also known as backbone cyclisation). This strategy is used to link the C- and N-termini together and results in the formation of cyclic homodetic peptides.
- Head-to-side-chain or side-chain-to-tail. The C-terminus (head) or the N-terminus (tail) is coupled to one of the side-chain groups forming a cyclic heterodetic peptide, such as depsipeptides or isopeptides.
- Side chain-to-side chain synthesis allows the formation of disulfide bridged peptides and stapled peptides.
To learn more about these methods, you can also visit our article on peptide cyclisation. Also, for recent examples of cyclic peptides synthesised in our laboratory, please see citations [3,4].
Benefits of cyclic peptides
Cyclisation enhances their biological and chemical properties, offering advantages over linear peptides. In particular, these include:
- Increased binding and specificity,
- Increased stability and resistance to enzyme degradation,
- and in certain cases, improved cellular uptake.
In addition, cyclic peptides occupy a unique position between small molecules and biologics, combining benefits of both. They provide alternatives for targeting “undruggable” protein targets, offering a promising option as therapeutics for drug discovery.
Cyclic peptide applications
Their applications are numerous, ranging from therapeutic drugs and food preservatives to pesticides in agriculture and valuable research tools. Below are some examples of applications in life sciences and drug discovery[5]:
- Cyclic peptide therapeutics (e.g., antibiotics, antiviral, cancer therapy) – Their interesting properties make them valuable candidates for therapeutic applications in drug discovery.
- Protein-protein interaction inhibitors/activators
- Cell-penetrating peptides
- Nanotechnology and drug delivery systems
- Mimics of protein structural motifs
- Biosensors
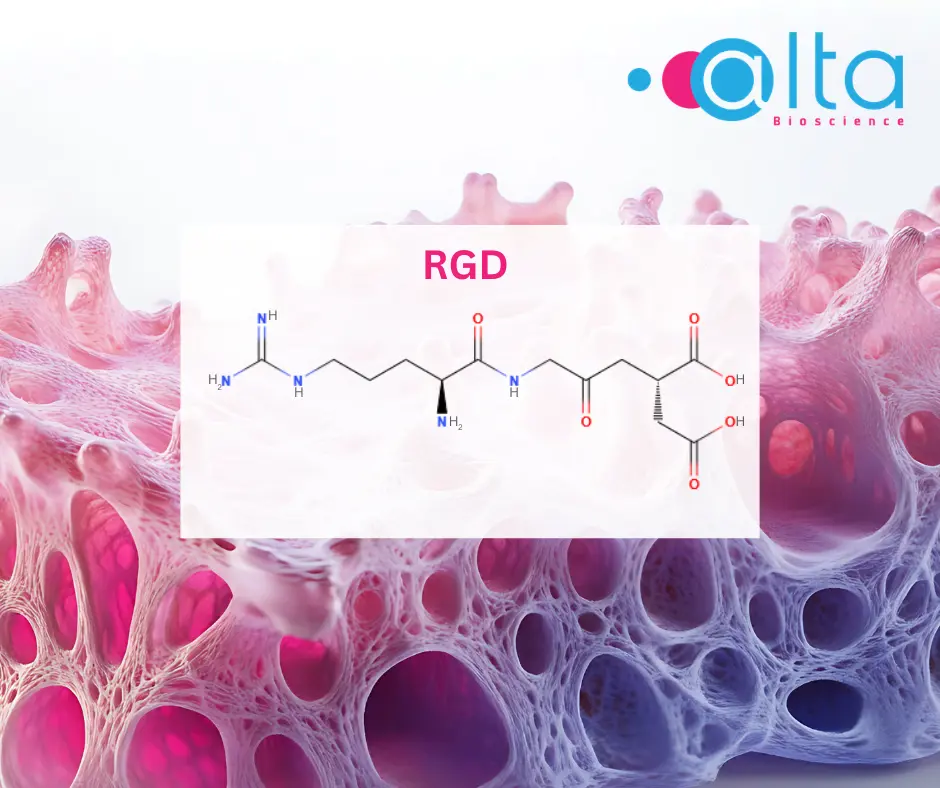
- Imaging and diagnostics: For instance, radiolabelled cyclic RGD (Arg-Gly-Asp), a peptide known to target overexpressed integrin αvβ3 in cancer cells, shows promising results as imaging probes for early cancer detection and non-invasive tumour monitoring.[6]
Get in touch
With over 50 years of expertise in synthesising constrained peptides for researchers worldwide and rigorous quality control in place, our custom peptide synthesis laboratory can provide the desired products with fast lead times. If you would like to find out more or discuss your requirements with our team, please contact us or email info@altabioscience.com.
Glossary of key terms
Biosynthesis pathways
Non-ribosomal cyclic peptides are peptides produced by microbial non-ribosomal peptide synthetases (NRPSs) as secondary metabolites. Contrary to ribosomal peptides, their synthesis is not limited to the genetic code so their structures are extremely varied.
Ribosomal peptides (also known as RiPP peptides) are peptides synthesised by ribosomes during the translation step of peptide synthesis. Ribosomes are macromolecular machines that read mRNA and assemble one amino acid at a time. After formation of the linear peptide chains, cyclisation occurs afterwards following post-translational modifications.
Types:
Depsipeptide (cyclic): A cyclic structure where the loop is closed by an ester/lactone group.
Dipeptide, also known as cyclodipeptide, are made up of two amino acids linked together by peptide bonds, forming a ring structure.[7]
Heterodetic peptide (cyclic): cyclic peptide containing amino acid residues but whose cyclisation has been achieved by forming a bond different than an amide, e.g. disulfide bridged peptides, depsipeptides etc.
Homodetic peptide (cyclic), a peptide where all the looped amino acid residues are linked by only amide bonds. For example, a peptide obtained by “head-to-tail” cyclisation between the C and N termini.
Isopeptide (cyclic): In this case, the loop is formed via an amide bond, but with an amino group not in the alpha position.
References
[1] CyclicPepedia (biosino.org)
[2] CycPeptMPDB
[3] Matthew Vassey, Rininta Firdaus, Akhmed Aslam, Lee M. Wheldon, Neil J. Oldfield, Dlawer A. A. Ala’Aldeen, Karl G. Wooldridge, G1 Cell Cycle Arrest Is Induced by the Fourth Extracellular Loop of Meningococcal PorA in Epithelial and Endothelial Cells, Cellular Microbiology Volume 2023, Article ID 7480033, 15 pages https://doi.org/10.1155/2023/7480033
[4] Hosam Alden Baksamawi, Alessio Alexiadis, Daniele Vigolo and Alexander Brill Platelet accumulation in an endothelium-coated elastic vein valve model of deep vein thrombosis is mediated by GPIbα—VWF interaction, Front. Cardiovasc. Med., 27 April 2023 Sec. Thrombosis and Haemostasis Volume 10 – 2023, https://doi.org/10.3389/fcvm.2023.1167884
[5] Choi JS, Joo SH. Recent Trends in Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol Ther (Seoul). 2020 Jan 1;28(1):18-24. doi: 10.4062/biomolther.2019.082. PMID: 31597413; PMCID: PMC6939695.
[6] Jiyun Shi, Shuang Liu, Clinical application of 99mTc-labeled peptides for tumor imaging: Current status and future directions, iRadiology, Volume 2, Issue 1, February 2024, Pages 17-34 https://doi.org/10.1002/ird3.55
[7] Wahyu Setia Widodo, Sonja Billerbeck, Natural and engineered cyclodipeptides: Biosynthesis, chemical diversity, and engineering strategies for diversification and high-yield bioproduction., Engineering Microbiology, Volume 3, Issue 1, 2023, 100067, ISSN 2667-3703, https://doi.org/10.1016/j.engmic.2022.100067.